Abstract
We and others have demonstrated that the immature megakaryocytes (MK) observed in the bone marrow (BM) from patients with myelofibrosis (MF) play a causative role in the establishment of this disease [reviewed in 1]. By leveraging scRNA-seq and other methods, recent studies have identified the existence of at least three megakaryocyte subpopulations, each one exerting different functions [2-4]: the most prevalent is that of platelet producing MK, the second is niche MK (defined here as hematopoietic stem cell (HSC)-supporting for clarity) and the third is classified as immune MK. In addition, the human fetus contains a fourth subpopulation, also defined niche MK, characterized by high expression of extracellular matrix genes, such as COL1A1 and COL3A1, and by a TGF-β signature, which is responsible for shaping the extracellular matrix of the organs during development. Platelet producing MK fall into the high ploidy class, express genes related to thrombopoiesis and are characterized by the CD42b/ARNTL markers. HSC-supporting MKs are mostly >=8N with high expression of IGF1and PF4, which regulate the growth of HSCs, and are characterized by the CD42b/MYLK4 markers. Immune MK are low ploidy with signatures of inflammatory response and myeloid leukocyte activation genes and are characterized as CD41/LSP1 cells. Lastly, niche MK, which are absent in adult BM, are characterized as CD42b/Col I and/or Col III cells.
We hypothesize that the immature morphology of the MK from the BM of MF patients flags changes in the relative frequencies of the different MK subpopulations and induction of the formation of niche MK, originally identified in the fetus. To test this hypothesis, we performed extensive confocal microscopy studies with MK-specific markers on the BM from three patients with MF (and three patients with essential thrombocythemia, ET, or with other malignancies but no fibrosis, non-MF, as control) and of the BM from Gata1low mice before (young) and after (old) onset of MF, using age matched wild type (WT) littermates as control (three mice/group).
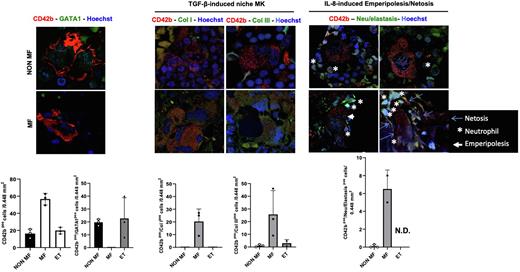
First we confirmed that the BM from MF patients contains greater numbers of MK than that from ET and non-MF patients and that these MF MK have an immature morphology and contain lower levels of GATA1 compared to controls (Fig. 1). We then demonstrated that MF MK are engaged in greater numbers in neutrophils emperipolesis associated with Netosis (Fig. 1), two events predicted to be triggered by the high bioavailability of interleukin-8 in the BM microenvironment of MF patients.
Third, we observed that platelet MK (CD42b+ARNTL+) are present in the BM from MF, ET and non-MF patients and from that of WT and Gata1low mice of all ages. By contrast, HSC-supporting MK (CD42b+MYLK4+) are observed in the BM from ET but not in that from MF. They are also observed in the BM from WT mice (both young and old) and from young Gata1low mice, but not in that from old Gata1low mice. Immune MK (CD42b+LSP1+) are instead observed in the BM from ET and non-MF patients but not in MF patients and in the BM from young and old WT mice and in that from young Gata1low mice but not in that from old Gata1low mice. Lastly, great numbers of the CD42b MK in the BM from MF patients, but not in that from ET and non-MF, contain Col I and III (Fig. 1). These cells are also present in the BM from old Gata1low mice but not in their age-matched WT littermates (data not shown) and may represent niche MK the differentiation of which is reactivated by the high TGF-β bioavailability in the BM from MF.
In conclusion, these results confirm the hypothesis that changes in frequency of MK subpopulations, rather than in MK maturity state per se, plays a causative role in the development of MF.
References: 1) Melo-Cardenas et al, Hematology/Oncology Clinics of North America, 2021 https://doi.org/10.1016/j.hoc.2020.11.004; 2) Sun et al, Blood 2021. 138: 1211; 3) Wang et al, Cell Stem Cells 2021. 28: 535; 4) Yeung et al, Blood Adv 2020. 4: 6204; 5) Pariser et al, JCI 2021. 131: e137377.
Figure 1: MK subpopulations differ in MF versus ET and individuals with hematological malignancies with no fibrosis. Confocal microscopy analyses confirms that there is an increase in CD42b+ MKs in MF, that MF CD42+ MKs lack GATA1 expression and express high levels of ColI and Col III and that a high percentage of them are engaged in neutrophil emperipolesis. Data were obtained on BM sections from MF and ET patients and from hematologic patients with no BM fibrosis (three patients/group).
Disclosures
Guglielmelli:Novartis, Abbvie: Other: Other member of advisory board, speaker at meeting. Vannucchi:Morphosys: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Migliaccio:Novartis: Research Funding; Dompe farmaceutici Spa R&D: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal